8. Atoms – Electrons, Protons and Neutrons
Atoms are the basis of chemistry. They are the basis for everything in the Universe as all matter is composed of atoms.
There are pieces of matter that are smaller than atoms – neutrons, electrons, and protons, they are the 3 basic parts of an atom. What are electrons, protons, and neutrons? A picture works best.
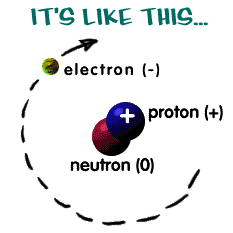
There are three pieces to an atom – electrons, protons, and neutrons. As you know, there are over 100 elements in the periodic table. The thing that makes each of those elements different is the number of electrons, protons, and neutrons. The protons and neutrons are always in the center of the atom. Scientists call the center of the atom the nucleus. The electrons are always found whizzing around the center in areas called orbitals. Electrons have a negative charge, protons have a positive charge. If the charge of an entire atom is “0”, that means there are equal numbers of positive and negative pieces, equal numbers of electrons and protons. The third particle is the neutron. It has a neutral charge (a charge of zero).